In an vital and spectacular new paper, Parker Rogers appears at what occurs when the FDA deregulates or “down-classifies” a medical machine kind from a extra stringent to a much less stringent class. He finds that deregulated machine sorts present will increase in entry, innovation, as measured by patents and patent high quality, and reduces in costs. Security is both negligibly affected or, within the case of merchandise that come underneath potential litigation, elevated.
After shifting from Class III (excessive regulation) to II (reasonable), machine sorts exhibited a 200% improve in patenting and FDA submission charges relative to manage teams. Patents filed after these occasions have been additionally of considerably larger high quality, as measured by a 200% improve in acquired citations and market valuations. These results don’t spill over into related machine sorts.1 For Class II to I deregulations, the speed of patent filings elevated by 50%, although insignificantly, and the standard of patent filings exhibited a major 10-fold enchancment, suggesting that litigation higher promotes innovation.
…Down-classification yields appreciable advantages, because the proponents of deregulation would predict, however what of product security? Maybe counterintuitively, I discover that deregulation can enhance product security by exposing companies to extra litigation. Regardless of some adversarial occasion charges rising after Class III to II occasions (albeit insignificantly), Class II to I occasions are related to considerably decrease adversarial occasion charges.3 My evaluation of patent texts additionally reveals that inventors focus extra on product security after deregulation. These outcomes counsel that litigation encourages product security greater than regulation…
Some background. Medical gadgets are regulated underneath three classes. New varieties of gadgets (new, not essentially excessive threat) are extremely regulated Class III gadgets which should undergo a pre-market approval course of to show security and efficacy (like new medicine). The pre-market approval course of is time-consuming and costly nevertheless it comes with one vital profit, federal preemption of state tort motion, i.e. these gadgets are shielded from product legal responsibility. Class II gadgets are gadgets which might be judged to be considerably equal to an already authorized machine–proving equivalence additionally takes money and time nevertheless it’s much less onerous than proving security and efficacy de novo. Observe that machine producers usually make their gadgets much less modern to allow them to be authorized as Class II gadgets quite than as Class III gadgets. Class II gadgets are largely additionally protected in opposition to tort litigation. Class I gadgets aren’t FDA-approved and are topic to tort litigation.
As expertise develops with new gadgets such that new gadgets transform not particularly dangerous, the FDA typically deregulates or down-classifies these gadgets from Class III to Class II or from Class II to Class I. Rogers research these down-classifications by evaluating what occurs to the down-classified machine class to a management group of comparable gadgets that weren’t down-classified. The management group is crucial and Rogers reveals that his outcomes are strong to defining the management group in quite a lot of believable methods. A few of the key outcomes are proven within the following determine:
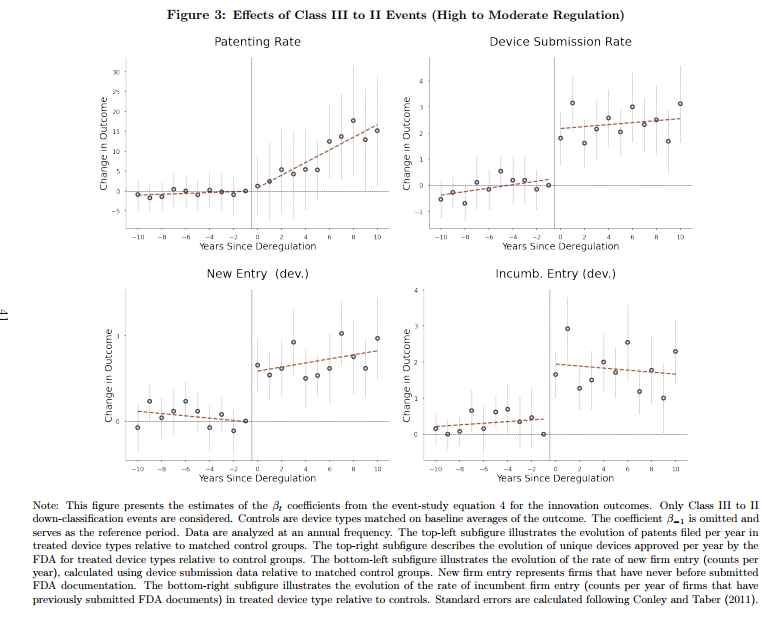 Properly we see that machine submissions and new entry happen in a short time as soon as a tool is down-regulated which signifies that companies have concepts and merchandise on-the-shelf however they’re dissuaded from coming into the market by the onerous pre-market approval course of. More than likely, these are merchandise and companies which produce gadgets for the European market which tends to be much less regulated and so they enter the US market solely when prices are diminished. Patenting additionally will increase within the down-regulated machine class and–precisely as one would count on–this takes extra time.
Properly we see that machine submissions and new entry happen in a short time as soon as a tool is down-regulated which signifies that companies have concepts and merchandise on-the-shelf however they’re dissuaded from coming into the market by the onerous pre-market approval course of. More than likely, these are merchandise and companies which produce gadgets for the European market which tends to be much less regulated and so they enter the US market solely when prices are diminished. Patenting additionally will increase within the down-regulated machine class and–precisely as one would count on–this takes extra time.
Security declines non-significantly if in any respect from Class III to Class II deregulations and will increase for Class II to Class I deregulations. That makes the welfare comparisons straightforward as a result of deregulation seems to be all profit and no value. Observe, nonetheless, that I’ve all the time argued that medicine and gadgets are literally too protected–that, is we may save extra lives on internet by approving extra medicine and gadgets even when security went down. That’s a tough promote, nonetheless, nevertheless it’s clearly true that given the outcomes right here we must always decontrol or down-classify many extra merchandise even when security declined on the margin. An excessive amount of security is dangerous. That’s additionally the upshot of my paper on off-label prescribing which reveals that it’s usually the FDA-unapproved off-label use which is the gold-standard therapy in fast-paced fields of drugs.
Rogers argues that security will increase for Class II to Class I deregulations as a result of legal responsibility is a stronger deterrent on the margin than regulation (and he gives some proof for this view in that security will increase extra amongst bigger companies which might be much less judgment proof than small companies). With out denying that mechanism my view is that innovation itself will increase security. As I famous above, medical machine manufactures usually don’t use the most recent know-how of their merchandise as a result of this might threaten the “substantial equivalence” check so that you get gadgets which might be really much less protected and likewise extra pricey to fabricate than vital. In essence, substantial equivalence anchors new applied sciences to previous applied sciences thus stopping motion, even motion in the direction of security and decrease costs.
Rogers additionally has a superb and weird paper (with Jeffrey Clemens) on directed innovation in synthetic limbs because of the civil battle! That paper and this one present an actual give attention to digging deep into the info to unearth vital and weird sources of perception. N.B.! Parker Rogers is on the job market.
Addendum: See my many earlier posts for extra helpful references on the FDA, particularly Is the FDA Too Conservative or Too Aggressive.
The put up FDA Deregulation Will increase Security and Innovation and Reduces Costs appeared first on Marginal REVOLUTION.




